Are you struggling to understand Gibbs free energy? Do you need practice problems and worksheets to solidify your understanding of this important concept? Look no further! In this article, we will provide you with a comprehensive guide on Gibbs Free Energy Worksheets and offer a collection of worksheets to help you master this topic. Whether you’re a student studying thermodynamics or a curious learner seeking to deepen your knowledge, these resources will be invaluable to your learning journey.
Gibbs Free Energy
Gibbs free energy, named after American scientist Josiah Willard Gibbs, is a thermodynamic property used to determine the spontaneity and equilibrium of a chemical reaction. It combines the concepts of enthalpy (heat transfer) and entropy (disorder or randomness) to provide insights into the direction in which a reaction will proceed.
The Significance of Gibbs Free Energy
Gibbs free energy is a crucial concept in thermodynamics as it helps predict whether a chemical reaction will occur spontaneously under certain conditions. It determines whether a system is at equilibrium or if it will proceed in the forward or reverse direction.
Understanding the Gibbs Free Energy Equation
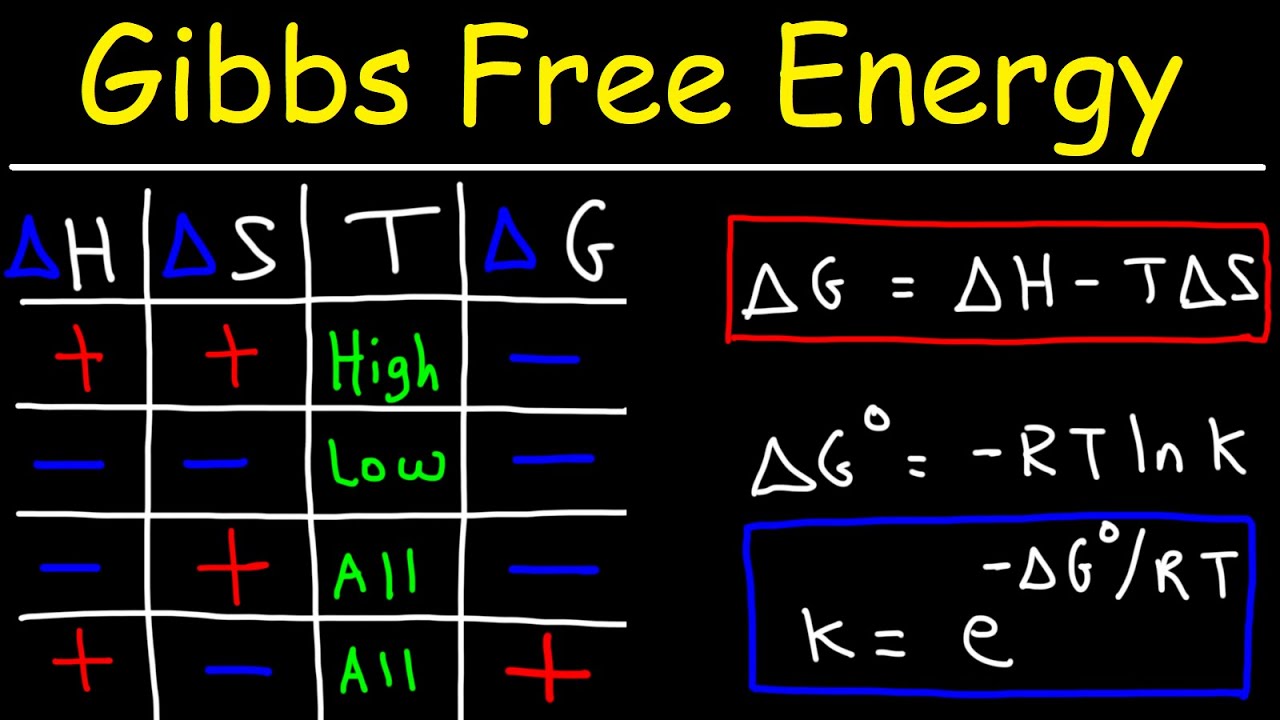
The Gibbs free energy equation combines the enthalpy change (ΔH) and the entropy change (ΔS) of a system:
ΔG = ΔH – TΔS
Where:
- ΔG is the Gibbs free energy change
- ΔH is the enthalpy change
- ΔS is the entropy change
- T is the temperature in Kelvin
Calculating Gibbs Free Energy
To calculate the Gibbs free energy change, you need to know the enthalpy change and the entropy change of the system. By substituting these values into the Gibbs free energy equation, you can determine the direction and extent of the reaction.
Spontaneity and Gibbs Free Energy
Gibbs free energy provides information about the spontaneity of a reaction. If ΔG is negative, the reaction is spontaneous and will proceed in the forward direction. Conversely, if ΔG is positive, the reaction is non-spontaneous and will require an external energy source to proceed.
Standard Gibbs Free Energy Change
The standard Gibbs free energy change (ΔG°) is the Gibbs free energy change under standard conditions, which include a temperature of 298 K (25°C), a pressure of 1 bar, and a concentration of 1 M for all species involved in the reaction. It allows for the comparison of different reactions under the same conditions.
Relationship Between Gibbs Free Energy and Equilibrium Constant
The Gibbs free energy change is related to the equilibrium constant (K) of a reaction through the equation:
ΔG° = -RT ln(K)
Where:
- R is the gas constant (8.314 J/(mol·K))
- T is the temperature in Kelvin
- ln denotes the natural logarithm
Gibbs Free Energy and Temperature
Temperature plays a crucial role in determining the spontaneity of a reaction. As the temperature increases, the magnitude of the entropy term (TΔS) in the Gibbs free energy equation becomes more significant. Thus, a reaction that may be non-spontaneous at low temperatures can become spontaneous at higher temperatures.
Factors Affecting Gibbs Free Energy
Several factors influence the Gibbs free energy of a system, including temperature, pressure, concentration, and the presence of catalysts. Understanding these factors is essential for predicting and controlling the direction and spontaneity of chemical reactions.
Applications of Gibbs Free Energy
Gibbs free energy finds applications in various fields, including chemistry, biochemistry, and material science. It is particularly useful in understanding and predicting phase transitions, chemical equilibrium, and the feasibility of chemical reactions.
Gibbs Free Energy Worksheets
To reinforce your understanding of Gibbs free energy, we have prepared a collection of worksheets. These worksheets provide practice problems and scenarios for you to apply the concepts and equations associated with Gibbs free energy. They cover topics such as calculating ΔG, determining spontaneity, and relating Gibbs free energy to equilibrium constant. You can access these worksheets by following the link below.
Conclusion
In conclusion, Gibbs free energy is a fundamental concept in thermodynamics that helps us understand the spontaneity and direction of chemical reactions. By grasping the key principles and equations related to Gibbs free energy, you can make accurate predictions and analyze various chemical processes. Remember to practice with the provided worksheets to reinforce your knowledge and enhance your problem-solving skills.
FAQs
1. What is the Gibbs free energy?
The Gibbs free energy is a thermodynamic property that determines the spontaneity and equilibrium of a chemical reaction. It combines the concepts of enthalpy and entropy to provide insights into the direction in which a reaction will proceed.
2. How is Gibbs free energy related to spontaneity?
Gibbs free energy is directly related to spontaneity. If the Gibbs free energy change (ΔG) is negative, the reaction is spontaneous and will proceed in the forward direction. Conversely, if ΔG is positive, the reaction is non-spontaneous and requires an external energy source.
3. Can Gibbs free energy be negative?
Yes, Gibbs free energy can be negative. A negative ΔG indicates that the reaction is spontaneous and can occur without the need for external energy input.
4. What is the significance of standard Gibbs free energy change?
The standard Gibbs free energy change (ΔG°) allows for the comparison of different reactions under standard conditions. It provides a reference point for assessing the feasibility and spontaneity of reactions.
5. Where can I find more resources on Gibbs free energy?
For more resources on Gibbs free energy and related topics, you can refer to textbooks on thermodynamics, online educational platforms, and scientific journals specializing in chemistry and thermodynamics. Additionally, you can access the provided worksheets to further enhance your understanding and problem-solving skills.
Read : Tink Million Free Download